Session (3)
Step 2 Acquiring Evidence (Search)
- Finding research is step two of the EBP process.
- After a clinical question has been formed, and the population has been identified, research and literature reviews must be obtained from reliable sources and then appraised for validity and reliability.
- Commonly used sources include journals, clinical practice guidelines, and textbooks.
- Using online sources and filters to narrow search results is the most efficient method of researching a topic.
- Each keyword from the PICOT question is used in the search.
- Many journals can be accessed free via the National Institute of Health and the National Library of Medicine.
- Clinical practice guidelines can be accessed via the public resource website National Guideline Clearinghouse.
- In the research and literature review process, the quality of the evidence is examined, as well as the level of evidence. The quality of the evidence is based on how confident a researcher is that the information gained from a study is adequate enough to help formulate a recommendation.
- The level of evidence is based on the type of source, whether it is a study, literature review, clinical practice guideline, or expert panel opinion.
- There are six levels of evidence. The levels of evidence range from Level I to Level VII, with Level I evidence being the strongest level of evidence.
- When researching a clinical question, the researcher must determine the strength of the literature in terms of quality, quantity, and consistency.
- It is often difficult for a new researcher to evaluate the level of research.
- Do not be afraid to ask for help in interpreting the information and statistical data in the research studies.
- It is necessary to correctly appraise the literature to ensure the scientific validity and applicability to the selected patient population.
Three core questions should be asked when evaluating literature.
- What are the actual results of the study?
- Are the results trustworthy and credible?
- Will the results help me in caring for my patient population?
- The Critical Appraisal Skills Program (CASP) is a program recommended by Quality and Safety Education for Nurses (QSEN) used to train people to perform critical appraisals.
- CASP’s goal is to help clinical decision-makers understand scientific evidence around the world. Nurses who want to implement research evidence into their own practice can utilize CASP. The website offers eight appraisal tools, includes checklists for nurses to use while reading the research, and offers workshops to help nurses better understand the research.
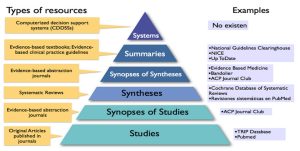
Identifying Information Resources

- After successfully formulating the clinical question in Step 1, you need to find relevant evidence to help answer your question. You will often need to consult several types of information resources.
- Information resources generally fall into three categories and are used in sequential order depending on need and applicability to your question.
The three categories are:
- Background Resources
- Filtered Resources
- Unfiltered Resources
- Note that some resources can contain both background information and filtered information. Some background resources are also filtered resources and may apply to a variety of question types. An example of this is DynaMed, which offers background information on a variety of conditions and treatments, and contains filtered information to aid in clinical decision-making.
- In this step, we will identify different types of resources, as well as some search strategies for using those resources.
Background Information Resources
- Background questions are meant to:
- Obtain general knowledge
- Learn about a new condition or topic
- Refresh your knowledge on a subject
- When you have a background question, background information resources are a great first stop. The goal of background resources is to provide an overview of a disease, condition, concept, or process and describe what is generally known and accepted. For example: measles has been nearly eradicated, but there are periodically outbreaks among unvaccinated populations.
- If you need to refresh your knowledge of the clinical presentation, diagnosis, etc. of measles, a background resource would be the best place to start.
- Background information resources can include textbooks and online resources. Most background resources will cite primary journal articles as evidence, so pay attention to the publication years of both the resource itself and the articles cited to help you determine whether the information is up-to-date.
Examples of Background Resources:
- DynaMed
- AccessMedicine
- Harrison’s Principles of Internal Medicine
- Briggs Drugs in Pregnancy and Lactation
- Trissel’s Handbook on Injectable Drugs
- Rang and Dale’s Pharmacology
- Adam and Victor’s Neurology
- AACN Essentials of Critical Care Nursing
- Canine and Feline Geriatric Oncology
Filtered Information Resources
- Foreground questions are meant to:
- Help with a decision on a course of action for a patient (diagnosis, treatment, etc.)
- Assist with a problem when you want to base your decision on the best available evidence.
- If you have a foreground question, consult a filtered resource.
- Filtered resources contain synthesized information. This means that clinical experts and subject specialists have posed a question and then synthesized the available primary studies – such as clinical trials – to make conclusions based on the available research. These resources are helpful because all available literature has been searched and their results have been evaluated to provide an answer to a clinical question. Make sure to evaluate how recently the synthesis was updated or revised – you may find that you need to find more evidence that is recent.
- Filtered information resources cover not all topics. If your topic is not covered, you will need to search the primary or unfiltered literature.
Examples of Filtered Information Resources:
- DynaMed
- Micromedex
- Cochrane Library
- Clinical practice guidelines, which you can find in the TRIP database or by searching the organization’s website
- Look for these resources and more at the Health Sciences Library’s homepage, hsl.lib.umn.edu.
Unfiltered Information Resources
- If a filtered resource does not cover a topic, does not apply to your patient, or you want to see what is new since the filtered resources were last updated, you can turn to unfiltered resources – often known as “primary literature.”
- When using primary literature, it is up to you to assess its quality, validity and applicability to your patient. Likewise, searching the primary literature can require more time and expertise, which is why filtered resources are a good place to get started. Every primary literature database is a little different. Learn about the database that you are searching and how best to search it. Librarians can help you with this.
Examples of Unfiltered Information Resources:
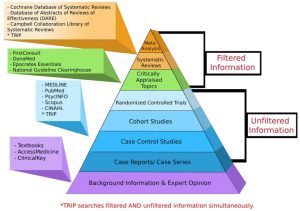
Evidence Hierarchy: What is the Best Evidence?
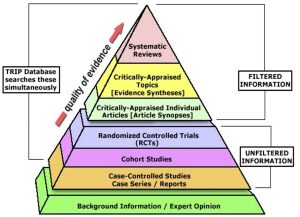
- Now that we have learned about the different types of resources – filtered, unfiltered, and background – let us look at the evidence hierarchy (also known as the levels of evidence).
- The evidence hierarchy pyramid is a visual representation of the strength of different research study designs. It can be helpful to think about evidence as a pyramid – not all study designs and resource types are created equal.
Filtered Information
- At the top of the pyramid, we have filtered information – this includes systematic reviews, meta-analyses, and evidence syntheses; practice guidelines; and critically appraised topics found in clinical resources. This type of information has used a high-quality methodology to synthesize primary resources – meaning that they have searched for available primary literature and evaluated its validity to provide answers to specific clinical questions. It is important to remember that the quality and reliability of filtered information can only be as good as the primary literature it includes.
Unfiltered Information
- In the middle of the pyramid, we have unfiltered information – this is known as primary literature. These are individual experimental study designs. A randomized controlled trial is considered the highest quality individual study design, followed by cohort studies and case-controlled studies. We will discuss these study designs in more detail later in the tutorial.
Background Information
- At the base of the pyramid, we have background information and expert opinion. Background information is not typically used in making complex clinical decisions, but can be helpful in defining parts of your clinical question.
Making Search Decisions
- Does background information on your topic answer your question?
- For example, are you looking for presentation information, a differential diagnosis list, or types of therapies? Try a background resource, such as DynaMed, ClinicalKey or AccessMedicine.
- Are you trying to decide on the best course of action (for diagnosis, treatment, etc.) and want to incorporate recent, reliable evidence into your decision?
- Consult a filtered resource, such as DynaMed, Cochrane Library, or Micromedex.
- Were you unable to find an answer to your question in a filtered resource, or want to find new original research studies?
- Try an unfiltered resource, such as PubMed, PsycINFO, or CINAHL.
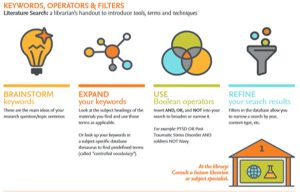
Search Terms
- When searching databases, you will need to determine which keywords to include in your search to help find relevant information. When it comes to keywords, simple is best. Search like a Neanderthal and not like a novelist. Select one keyword to describe the most important parts of your patient/problem and intervention.
- Keyword selection is a skill that takes practice and repetition to develop. Be flexible and gentle with yourself – you may need to try a few different times to get it right!
Example: Is vitamin c an effective treatment for the common cold?
Step 1 – Remove natural language and common words:
If we remove words like is, an, and the, we are left with: Is vitamin c an effective treatment for common cold?
Step 2 – Remove unnecessary words
Databases are not very good at reading your mind – they will search for all words you enter.
Think of what words might be unnecessary and narrow your search down too much. For example, effective treatment can be left out of your search, because any article that discusses vitamin c and the common cold will almost certainly be about using vitamin c as a treatment or prevention. Additionally, we can eliminate common – if we include “common” in our search, we will be excluding any articles that use cold instead of common cold.
- If we remove words like is, an, and the, we are left with: Is vitamin c cold?
Step 3 – Add/remove search terms as needed to narrow results
- Look at your search results and add or remove concepts as needed. Getting too many results? Add another concept to narrow your search. Not getting enough results? Remove a concept that may be eliminating too many results.
Step 4 – Try different keywords and combinations
Reassess! Be flexible and try different synonyms or combinations of keywords to see what works and what does not.
Levels of Evidence
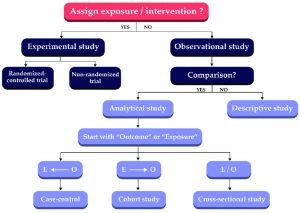
- In Step 2: Acquire, we introduced the Evidence-Based Pyramid. When you are looking for an article or resource that is appropriate to answer your clinical question, you want to look for the highest level of evidence that is available to you. At the top of the pyramid are systematic reviews, but a systematic review may not have been written about your topic yet, so you might end up with a randomized controlled trial (RCTs) instead.
- For some topics, you may not be able to find an RCT. Consider fields like obstetrics and pediatrics, where it may not be ethical to randomize patients to receive an experimental treatment or no treatment at all. For those fields, the highest level of evidence you may be able to find to answer your question is an observational study, such as a cohort study or a case-controlled study.
- It is important to recognize that the evidence pyramid is not rigid or prescriptive; think of it as a general guide to the reliability of evidence and its speed of use. For example, some systematic reviews can be of poor quality or inconclusive in their findings, and in those cases you may be better off using a well-designed RCTs. It is important to always assess the quality of the individual study.
- Typically, the higher your article is on the pyramid, the more reliable the evidence to answer your question. At the end of this section, there is a list of definitions of types of studies.
Research Designs
A research design is a strategy for answering your research question using empirical data. Creating a research design means making decisions about:
- Overall research objectivesand approach
- The type of research design one will use
- The sampling methodsor criteria for selecting subjects
- The data collection methods
- The procedures one will follow to collect data
- The data analysis methods
Characteristics of Research Design
- Reliability
Different types of research are conducted regularly. In such research, the researcher expects the research design to formulate questions that evoke similar results every time. A good research design is reliable to satiate the researcher’s needs to generate the same results every time.
- Validity
There are many ways to measure the results of research. However, with the help of a good research design, a researcher can select the right measuring tools that help in gauging the research results and align them with the research objectives to measure its success or failure. Therefore, the research design’s measuring tools must be valid and reliable enough to generate favorable results.
- Generalized
A good research design draws an outcome that can be applied to a large set of people and is not limited to sample size or the research group. The more applicable the research results are, the more the chances of it being accurate. Therefore, a good research design helps prove the research’s relevance and accuracy.
- Neutrality
At the start of every research, a researcher needs to make some assumptions that will be tested throughout the research. A proper research design ensures that the assumptions are free of bias and neutral. Furthermore, the data collected throughout the research is based on the assumptions made at the beginning of the research.
Different Approaches of Research Design
A researcher must be well versed in different types of research design. Moreover, a clear understanding of different research design types helps choose the right technique that incites favorable outcomes. Research design is broadly divided into quantitative and qualitative research design.
A: Quantitative research design
Quantitative research design aims at finding answers to who, what, where, how, and when through the course of research. Moreover, the outcome of the quantitative research is easy to represent in the form of statistics, graphs, charts, and numbers.
B: Qualitative research design
Qualitative research design aims at answering the how and why. It uses open-ended questions and helps the subjects express their views clearly. Qualitative research is ideal for research that aim to understand peoples’ behavior and requirements.
Research designs:
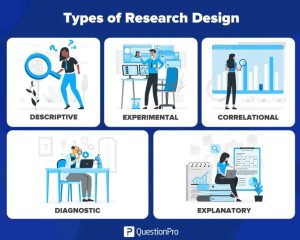
- Experimental design
The experimental design aims to look at a problem scientifically; that’s why it tries to establish a clear cause and effect of any event occurring in the research realm. Moreover, the research design tries to understand the impact of the independent variable on the dependable variable. As a result, this research is used to solve issues that try to analyze independent variables and their effect on dependable variables or vice-versa. - Correlational design
Correlation research design establishes a relationship between two related variables. Over time, the researcher observes the variables and then draws conclusions based on them. As a result, this type of research design requires two types of variables to function to draw favorable results. - Descriptive design
It is a hypothesis-based method that defines the primary subject matter of the research and tries to analyze it using different assumptions and techniques. This type of research design uses data collection techniques like natural observation, case studies, and surveys to derive results. - Diagnostic design
Diagnostic research design examines the elements posing challenges to businesses and customers. The methodology strives to explore the reason behind an issue and find solutions to solve it. Furthermore, this research design tries to solve issues in a structured form that follows three phases- inception, diagnostic, and solution. - Explanatory design
In this research design, the researcher explores innovative business concepts and ideas with the help of different scientific tools and techniques. This research design is ideal for a business’s research and development department because it offers innovative and creative ideas to solve a business problem.
Types of Research Design
Systematic Reviews & Meta-Analyses
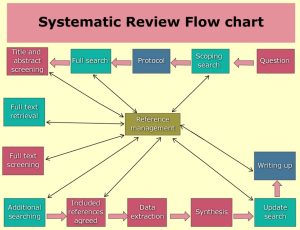
- A systematic review critically assesses and evaluates all research that addresses a particular research question and presents a synthesized summary of the literature. The researchers use a systematic methodology to search and screen the literature on a particular topic. Meta-analyses use statistical methods to combine the results of individual studies and synthesize the findings.
- The quality of a systematic review is only as good as the quality of the studies that are included. When evaluating this type of study, you want to assess the methodology of the search strategy and screening of articles to include, and the assessment by the authors of the included studies.
Randomized Controlled Trials (RCTs)
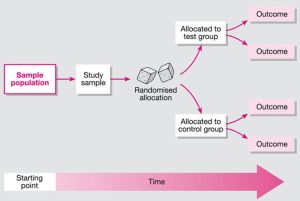
- A study design that randomly assigns participants to an experimental group (which receives the intervention) or a control group (which receives either a placebo or no intervention). The only expected difference between the two groups is the variable being studied.
- When critically appraising an RCT, you will evaluate elements of the study such as the allocation of participants, how similar the control group and the experimental group are, and the blinding of the participants and health care workers.
Cohort Studies
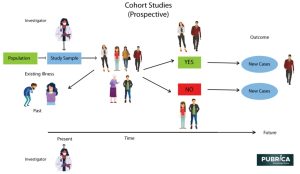
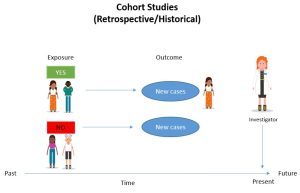
- A study type in which people who currently have a certain condition or receive a certain treatment are followed over time and compared with a group of people who are not affected by the condition or treatment.
- When critically appraising a cohort study, you will investigate how the cohort was recruited, if the exposure was accurately measured and bias was minimized, and if authors have identified and taken account of possible confounding factors.
Case Control Studies
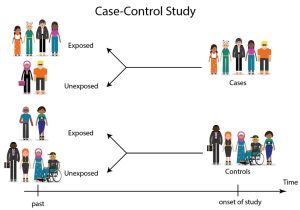
- An observational study of people with a disease (or other outcome variable) of interest and a control, comparison, or reference group of people without the disease. The two groups of people are compared to determine what can be attributed to the disease or outcome variable.
- Much like a cohort study, when critically appraising a case series you will examine whether the authors have minimized bias and properly addressed any potential confounding factors.
- Some study types are not named on the EBM Pyramid, but are important study designs for answering research questions:
Diagnostic Studies
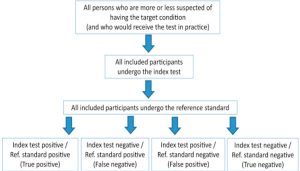
- This type of research focuses on estimating the sensitivity and/or specificity of a particular diagnostic test, and compares the test to the standard diagnostic test.
- When critically appraising this type of study you will want to determine if the new test was compared with an appropriate standard test, if all patients received both the new test and the standard test, and whether the health care workers administering the tests were properly blinded to the results of the standard test.
Clinical Practice Guidelines
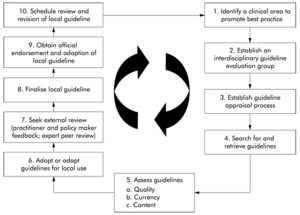
- Systematically developed statements of recommendations based on the best available evidence designed to assist practitioners with decisions about appropriate health care practices for specific clinical circumstances.
- The process used to develop guidelines involves a thorough evaluation of research evidence related to the outcomes of treatment or other health care procedures. Where research-based evidence is not available, consensus by experts forms the basis of the guideline.
- Clinical practice guidelines have been defined as decision tools to close gaps between current and optimal practice.
Economic Evaluation
- This type of study compares the costs and outcomes of healthcare interventions.
- When critically appraising an economic evaluation, you will determine if there is evidence that the new intervention or program is effective, if the effects of the intervention were measured appropriately, and were the costs valued in a credible manner.
Qualitative Studies
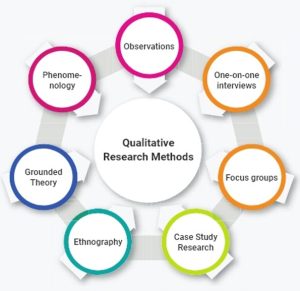
- Qualitative research aims to identify what matters most to patients or populations and how their experience can be improved. In public health, this type of research allows researchers to explore social and behavioral issues and explore other social or human problems.
- Critical appraisal of a qualitative study will determine if there was a clear aim for the research, if the qualitative methodology and research design were appropriate for the aims, and if the data analysis was sufficiently rigorous.